New insights and therapeutic approaches
Brain tumors and other tumors of the central nervous system (CNS) are the second most common cancer in childhood, accounting for about 24 percent of all cases, and are also the most common group of "solid" (i.e., solid, initially spatially limited) tumors.
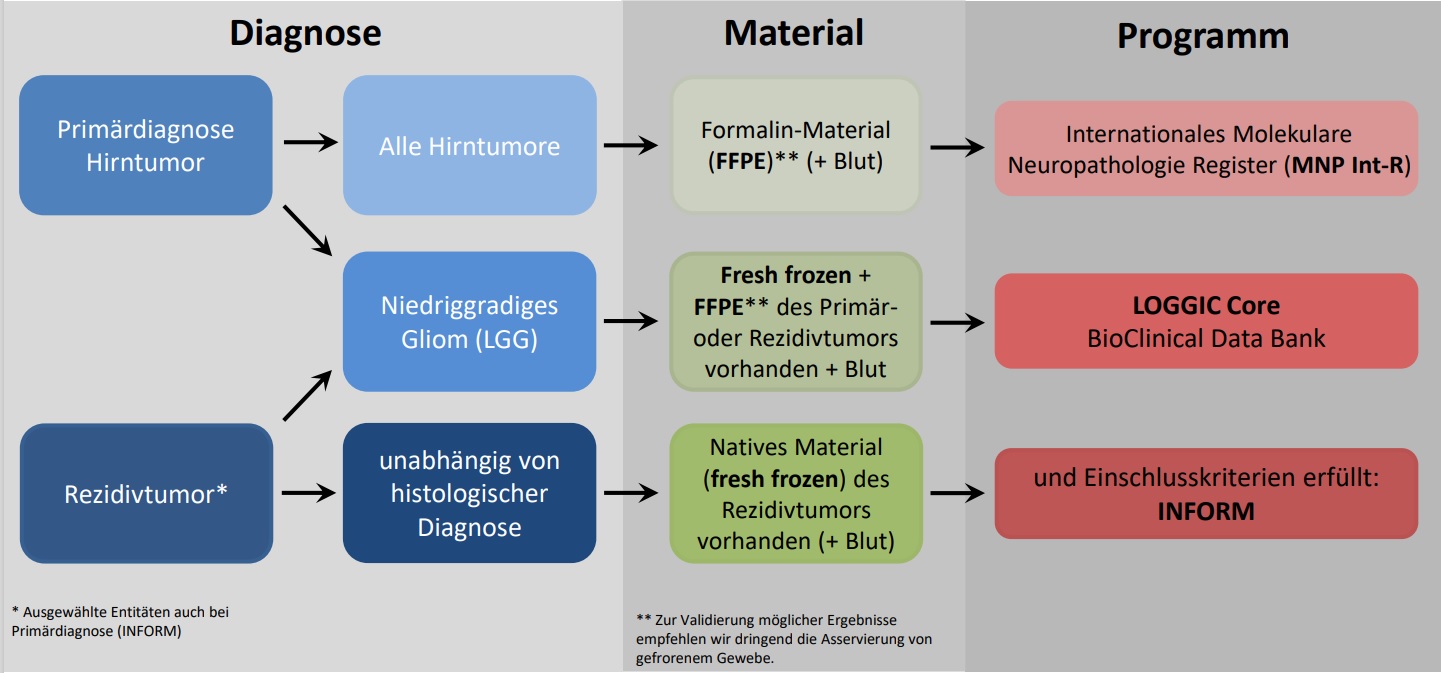
We always distinguish studies between clinical trials on the one hand, and diagnostic platforms and registry studies on the other. Clinical trials mean that a new treatment is tested for efficacy and tolerability, among other things. Diagnostic platforms offer molecular analyses of tumor material, while registry studies are observational studies that collect data on disease progression.
All of our clinical studies, as well as our diagnostic platforms and registry studies on solid and brain tumors, can be found here for review. We also have an overview of the diagnostic options, which can facilitate sorting.
Clinical Trials
Green indicates studies for which recruitment is still open. Red, on the other hand, are those for which recruitment has been completed. Diagnostic platforms and registry studies follow, as does our overview of diagnostic options.
Entrectinib
An open-label, dose-escalation, expansion phase I/II study of entrectinib in children with advanced or metastatic solid tumors or primary central nervous system tumors with/or without sufficient treatment options.
Selpercatinib
A phase I/II study of the oral RET inhibitor LOXO-292 in pediatric patients with advanced solid or primary central nervous system tumors with alterations in the RET gene.
Olaparib
PARP inhibitor olaparib for the treatment of progressive or relapsed solid tumors with alterations in homologous recombination (HRR).
Niraparib/ Dostarlimab
Unblinded, multicenter dose-escalation and cohort expansion study of niraparib and dostarlimab in pediatric patients with recurrent or progressive solid tumors (SCOOP).
Avapritinib
Phase I/II single-arm study to assess the safety, pharmacokinetics, and antitumor activity of avapritinib in pdiatric patients with tumors dependent on the KIT or PGFRA pathway (BLU-285-3101).
Diagnostic platforms and registry studies
Here are our diagnostic platforms and registry studies. Clinical trials have been listed above.

INFORM
INdividualized therapy FOr Relapsed Malignancies in Childhood: a unique multi-country genome sequencing program for children with cancer in Europe.
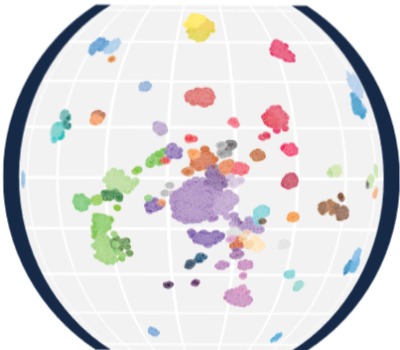
MNP Int R
The current MNP Int-R follow-up registry study aims to establish an international registry for the generation and coordination of molecular data in pediatric neuro-oncology.