[Translate to En:](Kopie 1)
(copy 1)
Development of preclinical models for testing targeted compounds
About 25 percent of children with cancer relapse despite intensive first-line therapy. The options for further treatment are then very limited. The search for new therapeutic options for children for whom established methods have not led to a lasting cure has been difficult to date. This is because molecular genetic data on tumor development and tumor resistance are limited, and for many cancer types and subtypes there are as yet no model organisms for preclinical testing of novel molecularly targeted agents .
The international ITCC-P4 project (Innovative Therapies for Children with Cancer Paediatric Preclinical Proof-of-concept Platform), which started in 2017 and is coordinated by DKFZ researchers at the KiTZ Heidelberg and the pharmaceutical company Eli Lilly, now aims to change that. ITCC-P4 is funded for five years with more than 16 million euros as an Innovative Medicines Initiative (IMI) in the form of a public-private partnership, with half of the funding coming from the EU (Horizon 2020) and half from the participating companies.
The overall goal of the initiative is to accelerate the development of new drugs for children with cancer and to develop new biomarkers. The tumor models developed in the participating academic research centers as part of the ITCC-P4 initiative will later be made available for the establishment of a comprehensive and sustainable platform on which drug tests can also be carried out in the future. It can be assumed that patient-specific tumor models will be more successful in achieving more meaningful results for the preparation of clinical studies than is currently possible with the usual tests on cancer cells in the culture dish or on genetically uniform mouse strains.
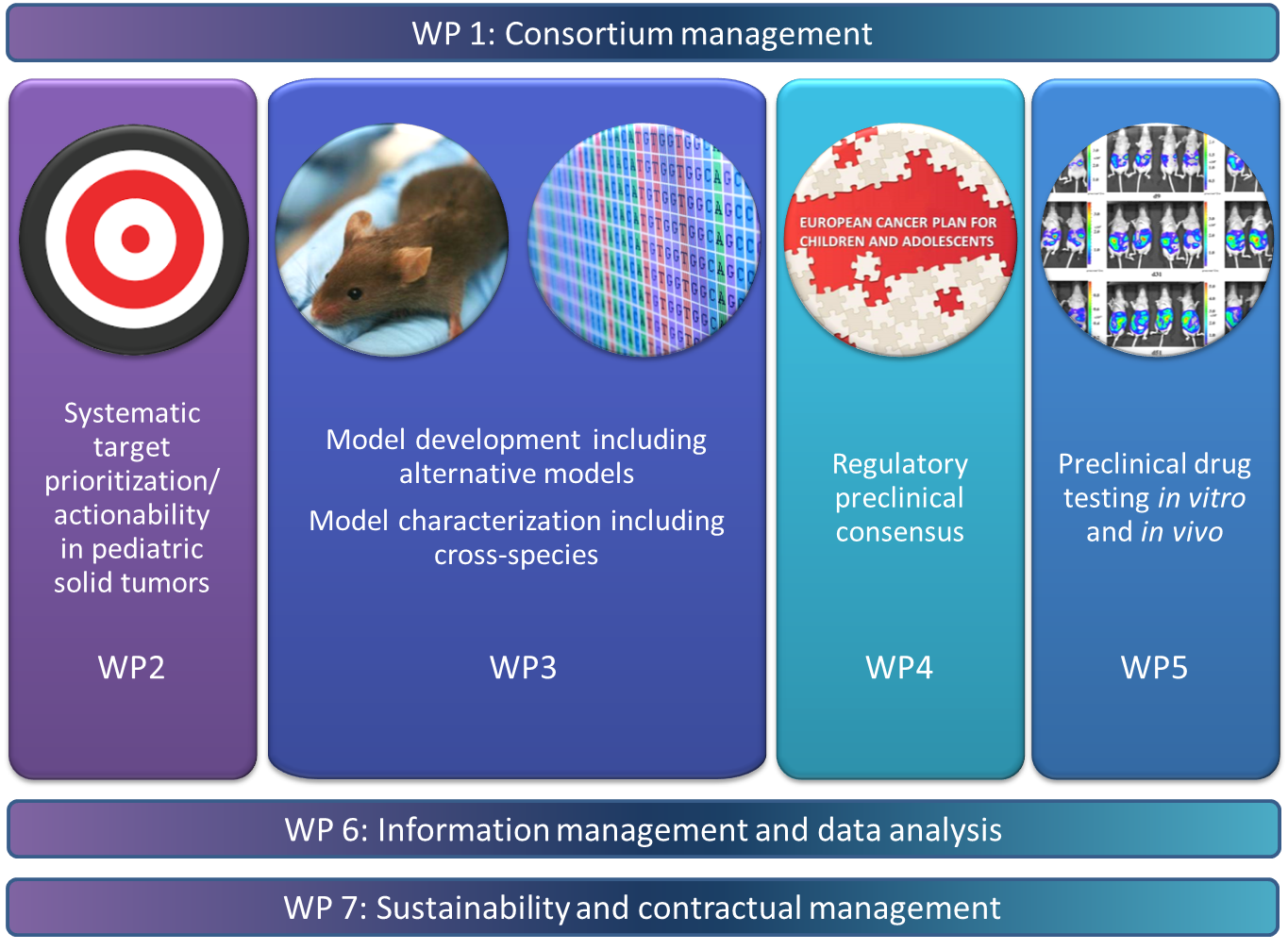
The approach
ITCC-P4 encompasses all the key steps needed to establish a sustainable, comprehensive, preclinical pediatric testing platform based on genetic information from more than 360 patient samples. The illustration summarizes the approach of the project.
-
Building a representative collection of approximately 400 patient in vitro and in vivo models as well as genetic mouse models of the most common pediatric solid high risk entities - with a significant share of recurrence models.
- Molecular characterization of the models as well as the associated primary tumor samples and germ line controls with state-of-the-art molecular diagnostic tools.
- Development of comprehensive preclinical data packages. These are necessary to use drugs in clinical trials for children with solid tumors.
- Prioritizing the development of pediatric medicines using the existing collection of molecular data for systematic targeting, followed by in vivo drug trials in disease models including pharmacokinetics and pharmacodynamics. Standard-of-care drugs are also being tested for all models in order to use them as a basis for the comparisons.
- Identification of suitable biomarkers for the future clinical stratification of patients across multiple entities.
-
In the long-term, the establishment of the ITCC P4 platform will ultimately fill a long-standing gap by enabling in-depth molecular characterization of high-risk pediatric malignancies coupled with standardized preclinical testing, thereby accelerating the development of more accurate and efficient drugs for this population. Developing this platform as a public-private partnership will create a collaborative model that can hopefully be extended to other types of cancer entities and patient populations.
Many of the most renowned European research facilites contribute to the ITCC-P4 consortium:
- DKFZ / KiTZ, Institute for Cancer Research (London, United Kingdom), European consortium for Innovative Therapies for Children with Cancer, Institute Gustave Roussy, Alleanza Contro il Cancro (Rom, Italy), Zürich University, Medizinische Universität Wien (MUW), Fundació Sant Joan de Déu Barcelona, Academic Medical Center (AMC) (Amsterdam, Netherlands), Children`s Cancer Research Institute Vienna, Institut Curie, Charité Berlin, Princess Máxima Center Utrecht
- Established small and medium-sized companies: EPO-Berlin-Buch GmbH, XenTech (Evry, France)
- Members of the “European Biopharmaceutical Enterprises (EBE“): PharmaMar (Madrid, Spain)
- Members of the “European Federation of Pharmaceutical Industries and Associations” (EFPIA): Lilly, Roche, Pfizer, Bayer, Charles River