(copy 1)
Pediatric glioma research
The research group "Pediatric Glioma Research", which corresponds to the DKFZ department of the same name, is headed by Dr. David T. W. Jones (PhD) and is part of the DKFZ research focus "Functional and Structural Genomics". The group's research focuses primarily on pediatric gliomas (both low-grade (LGG) and high-grade gliomas (HGG)) - the most common pediatric brain tumor. State-of-the-art genomic, epigenomic and functional technologies are being used to further understand the biology of these tumors.
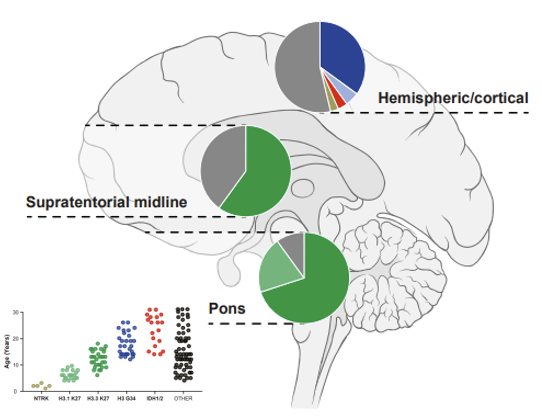
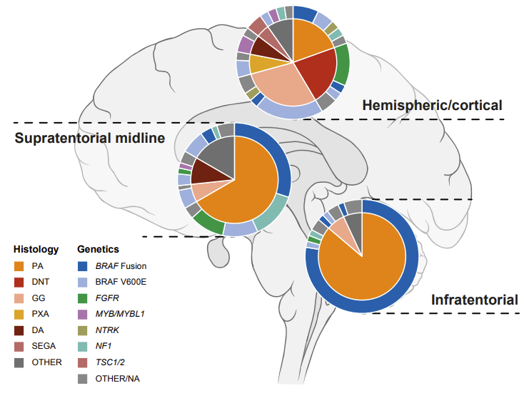
LGG are typically slow-growing with good survival rates, but this survival often comes at a significant cost to quality of life due to treatment effects and the location of the tumor. Recurrences are common, creating a heavy burden on patients and families.
HGG are genetically much more diverse and one of the most aggressive human cancers. Combined disruption of multiple cellular processes leads to rapidly dividing cancer cells that diffusely infiltrate the surrounding brain tissue, with a mostly fatal outcome.
Another important focus of the group is the coordination of two international molecular diagnostic programs: the INFORM study for identification of possible drug targets in high-risk pediatric cancers; and the MNP Int-R study to investigate and validate molecular analysis as a tool for accurate brain tumor classification.
Spotlight - Div. Jones
Multiomic neuropathology for improved diagnostic accuracy
A large prospective clinical validation program has demonstrated that the use of DNA methylation and gene panel sequencing to complement standard techniques is beneficial for pediatric brain tumor pathology and significantly improves diagnostic accuracy.
Gliomas are the most common CNS tumors in children and adolescents. Epigenetic analysis has become a growing analytical tool in pediatric brain tumor research to improve diagnostics and understand the molecular mechansims underlying their developmental progression. DNA methylation signatures together with integrated histopathology are now globally recognized diagnostic tools and are incorporated in the new guidelines of the WHO Classification of CNS tumors. We are looking at epigenetic modifications, such as DNA methylation patterns as well as histone modifications, which can alter gene expression. By identifying specific patterns of epigenetic changes in pediatric gliomas, we aim to develop new diagnostic tools and therapies to improve clinical management for children with these types of brain tumors. Our research has already yielded important insights into the biology of pediatric gliomas, and hold promise for the development of new effective treatments in the future.
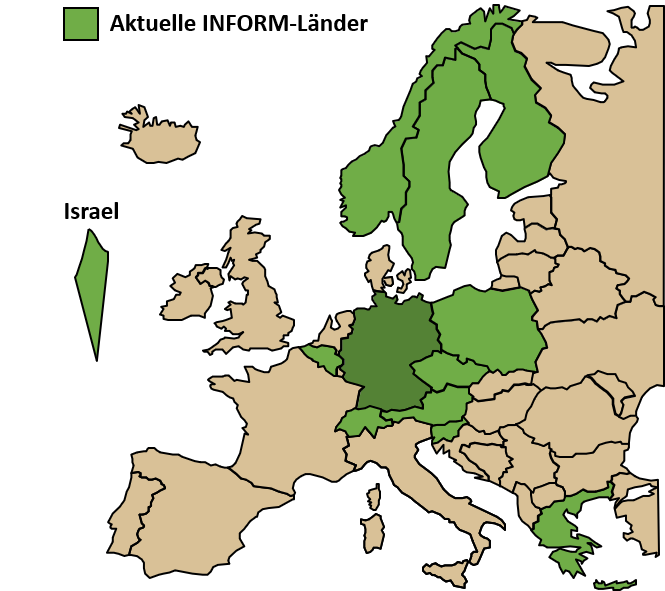
Our Division plays a key role in the organization of the international precision oncology program INFORM (INdividualized therapy FOr Relapsed Malignancies in children) by coordinating the molecular analyses and interpretation of patient data in real-time. As such, we provide access to comprehensive molecular profiling for high-risk pediatric cancer patients across Europe. The INFORM family covers 13 European countries, is running in more than 100 pediatric oncology research centers and has enrolled over 2500 relapsed/refractory pediatric cancer patients with approximately half of all cases coming from our international partners. The first clinical impact analysis demonstrated survival benefit for patients with very high priority targets who were treated with a matching drug. The study has further advanced and successfully implemented an ex vivo personalized drug sensitivity profiling platform under real-world conditions adding a new level of functional information to clinical decision making. This routine analysis is now in the transition to being reimbursed by health insurances in Germany and helps establishing such sequencing and screening platforms in other countries.
MNP Int-R is a follow-up registry study of the previous MNP 2.0 study that investigated the principle of DNA methylation signatures as part of a combined histological and molecular tumor classification to better and more accurately diagnose pediatric brain tumors at the time of primary diagnosis. This paved the way to add molecular diagnostics, including DNA methylation profiling and targeted gene panel sequencing, into routine diagnostics of primary CNS tumors in children and adolescents and integrated reference pathology, radiology and molecular results into a multilayered tumor diagnosis. MNP Int-R will follow the high diagnostic standard in an international setting and make a large impact on clinical disease management. At the same time, the continuous collection and analysis of data helps to identify yet undiscovered tumor types and sub-types, and discover the biology behind very rare molecular-defined tumor classes. Further development and training of the DNA methylation-based classification system helps to improve the molecular parameters and shapes long-term the design of future therapeutic studies influencing personalized precision treatment and new target therapeutic approaches.
Multiomic neuropathology improves diagnostic accuracy in pediatric neuro-oncology. Sturm D et al and Pietsch T*, Sahm F*, Pfister SM*, Jones DTW*. Nature Med. 2023. Accepted.
Amplification of the PLAG-family genes-PLAGL1 and PLAGL2-is a key feature of the novel tumor type CNS embryonal tumor with PLAGL amplification. Keck MK, Sill M, Wittmann A, Joshi P, Stichel D, Beck P, Okonechnikow K, Sievers P, Wefers AK, Roncaroli F, Avula S, McCabe MG, Hayden JT, Wesseling P, Øra I, Nistér M, Kranendonk MEG, Tops BBJ, Zapotocky M, Zamecnik J, Vasiljevic A, Fenouil T, Meyronet D, von Hoff K, Schüller U, Loiseau H, Figarella-Branger D, Kramm CM, Sturm D, Scheie D, Rauramaa T, Pesola J, Gojo J, Haberler C, Brandner S, Jacques T, Sexton Oates A, Saffery R, Koscielniak E, Baker SJ, Yip S, Snuderl M, Ud Din N, Samuel D, Schramm K, Blattner-Johnson M, Selt F, Ecker J, Milde T, von Deimling A, Korshunov A, Perry A, Pfister SM, Sahm F, Solomon DA, Jones DTW. Acta Neuropathol. 2022 Nov 27. doi: 10.1007/s00401-022-02516-2. Online ahead of print. PMID: 36437415
PATZ1 fusions define a novel molecularly distinct neuroepithelial tumor entity with a broad histological spectrum. Alhalabi KT, Stichel D, Sievers P, Peterziel H, Sommerkamp AC, Sturm D, Wittmann A, Sill M, Jäger N, Beck P, Pajtler KW, Snuderl M, Jour G, Delorenzo M, Martin AM, Levy A, Dalvi N, Hansford JR, Gottardo NG, Uro-Coste E, Maurage CA, Godfraind C, Vandenbos F, Pietsch T, Kramm C, Filippidou M, Kattamis A, Jones C, Øra I, Mikkelsen TS, Zapotocky M, Sumerauer D, Scheie D, McCabe M, Wesseling P, Tops BBJ, Kranendonk MEG, Karajannis MA, Bouvier N, Papaemmanuil E, Dohmen H, Acker T, von Hoff K, Schmid S, Miele E, Filipski K, Kitanovski L, Krskova L, Gojo J, Haberler C, Alvaro F, Ecker J, Selt F, Milde T, Witt O, Oehme I, Kool M, von Deimling A, Korshunov A, Pfister SM, Sahm F, Jones DTW. Acta Neuropathol. 2021 Nov;142(5):841-857. doi: 10.1007/s00401-021-02354-8. PMID: 34417833
Radiation-induced gliomas represent H3-/IDH-wild type pediatric gliomas with recurrent PDGFRA amplification and loss of CDKN2A/B. Deng MY, Sturm D, Pfaff E, Sill M, Stichel D, Balasubramanian GP, Tippelt S, Kramm C, Donson AM, Green AL, Jones C, Schittenhelm J, Ebinger M, Schuhmann MU, Jones BC, van Tilburg CM, Wittmann A, Golanov A, Ryzhova M, Ecker J, Milde T, Witt O, Sahm F, Reuss D, Sumerauer D, Zamecnik J, Korshunov A, von Deimling A, Pfister SM, Jones DTW. Nat Commun. 2021 Sep 20;12(1):5530. doi: 10.1038/s41467-021-25708-y. PMID: 34545083
The pediatric precision oncology INFORM registry: clinical outcome and benefit for patients with very high-evidence targets. van Tilburg CM et al. and Jones DTW, Molenaar JJ, Capper D, Pfister SM, Witt O. Cancer Discov. 2021 Aug 9:candisc.0094.2021. doi: 10.1158/2159-8290.CD-21-0094. Online ahead of print. PMID: 34373263
Infant high grade gliomas comprise multiple subgroups characterized by novel targetable gene fusions and favorable outcomes. Clarke M et al. and Ellison DW*, Jacques TS*, Jones DTW*, Jones C*.Cancer Discov. 2020 Apr 1. pii: CD-19-1030. doi: 10.1158/2159-8290.CD-19-1030. [Epub ahead of print]. PMID: 32238360
Molecular characteristics and therapeutic vulnerabilities across paediatric solid tumours. Jones DTW, Banito A, Grünewald TGP, Haber M, Jäger N, Kool M, Milde T, Molenaar JJ, Nabbi A, Pugh TJ, Schleiermacher G, Smith MA, Westermann F, Pfister SM.Nat Rev Cancer. 2019 Aug;19(8):420-438. doi: 10.1038/s41568-019-0169-x. Epub 2019 Jul 12. Review.PMID: 31300807
DNA methylation-based classification of central nervous system tumours. Capper D, Jones DTW, Sill M, Hovestadt V et al, and von Deimling A, Pfister SM. Nature. 2018 Mar 22;555(7697):469-474. doi: 10.1038/nature26000. Epub 2018 Mar 14. PMID: 29539639
- Anja Runge, PhD (Project Manager)
- Anna-Lisa Böttcher, PhD (Project Manager shared with Stefan Pfister)
- Andrea Wittmann (Technician)
- Kati Ernst (PhD Student shared with Marc Zuckermann)
- Jan Vaillant (PhD Student shared with Lena Kutscher)
- Laura von Soosten (PhD Student shared with Marc Zuckermann)
- Mirjam Blattner-Johnson, PhD (Postdoc)
- Michaela Keck, PhD (Postdoc)
- Kathrin Schramm, PhD (Postdoc)
- Barbara Jones, MD (Physician Scientist, UKHD)
- Elke Pfaff, MD (Physician Scientist, UKHD)
- Dominik Sturm, MD (Physician Scientist, UKHD)
- Karam Al-Halabi (MD Student)
- Ashwyn Augustine Perera (MD Student)